
An acid that is the molar concentration of the acid can give a proton that is the hydrogen ion concentration whereas a base can receive one, as per the Bronsted-Lowry theory of acids and bases.Concept of Henderson Hasselbalch Equation Assuming it does not analyze acid fragmentation and conjugate base hydrolysis, the total of the weak acid and conjugate base concentrations will remain constant.
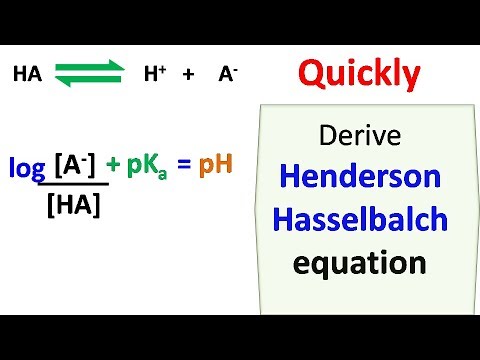
The drawback is that during titration, the Henderson Hasselbalch equation presupposes that the summation of the weak acid and conjugate base concentrations of a weak acid is constant.
The Henderson Hasselbalch equation drawback, we believe, is to blame for this strange and incorrect scenario. The inclusion of a strong base cannot logically describe the decrease in pH because all chemistry textbooks state that the pH values rise when a strong base is added to a weak acid solution. When the Henderson Hasselbalch equation is employed in an acid-base titration, it is undesirable since it yields the same result for any used concentration of weak acid and strong base, and after initiating the titration and applying the base, pH readings are lesser than the weak acid’s original pH. The pKa formula for weak acid or buffer can be used to get the equation. The formula can also be used to calculate the pH of a buffer solution or the equilibrium pH of an acid-base reaction. The Henderson Hasselbalch equation logically relates a solution’s measured pH with the acid’s pKa (that equals -log Ka). PK represents the acid dissociation constant. HA represents the molar concentration of the acid solution The Henderson Hasselbalch equation to estimate the hydrogen ion concentration in the solution isĪ – represent the molar concentration of conjugate base The acid is a proton giver, whereas the base is a proton receiver.

Strong acid and a weak conjugate base, or a strong base and a weak conjugate acid, are vividly present in the sample. How can you use the Henderson Hasselbalch equation to make a buffer solution? What is Henderson Hasselbalch EquationĪccording to biochemistry experts, the Henderson Hasselbalch equation is used to compute the concentration of hydrogen ions (acidity or alkalinity) in an aqueous solution containing a weak acid and its conjugate base.


 0 kommentar(er)
0 kommentar(er)
